Evaporation — physical-meteorological processes and observations
This unit provides one of the links between the water in all three phases that exists in the atmosphere and the water in reservoirs like ocean, lakes and rivers and soil water.
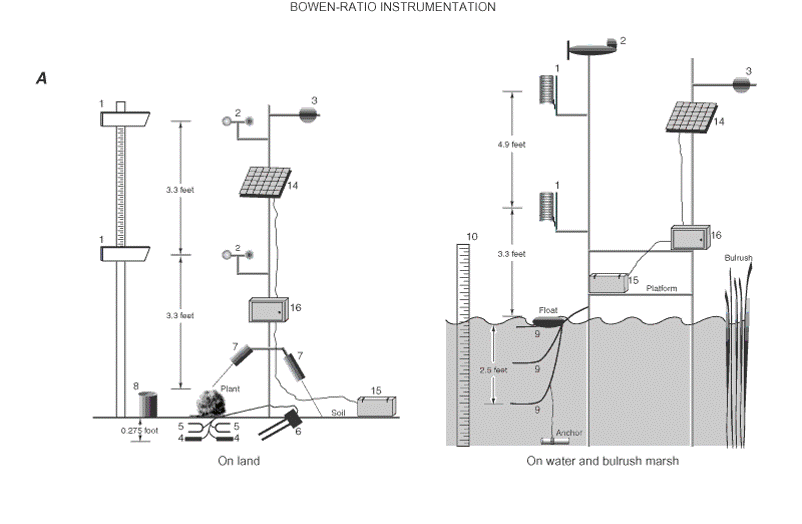
Goals
The goal of this unit is to describe one of the processes that adds water vapor to the atmosphere against the gravity force.
After successful completion of this unit students will be able to
- Calculate the evaporation from open water surfaces and bare soil
- Determine the energy required for evaporation
- Explain various methods to perform evaporation measurements
Students’ tasks
- Read Dingman’s Chapters 3.3 to 3.3.2 and 6 to 6.4 (included)
- Watch this movie by Waikato Earth and Ocean Sciences master’s student Tehani Kuske
- Watch this video of a worked problem
- Watch this video of a worked problem
- Fill out the questionnaire prior to Thursday 2359 Alaska time
- Solve the tasks assigned at your class level in this Unit 16 Applications sheet and submit the scanned results by Thursday 2359
FAQ
Q: The book said that evaporation from a large pan is less than from a small pan. Why?
A: Watch this video.
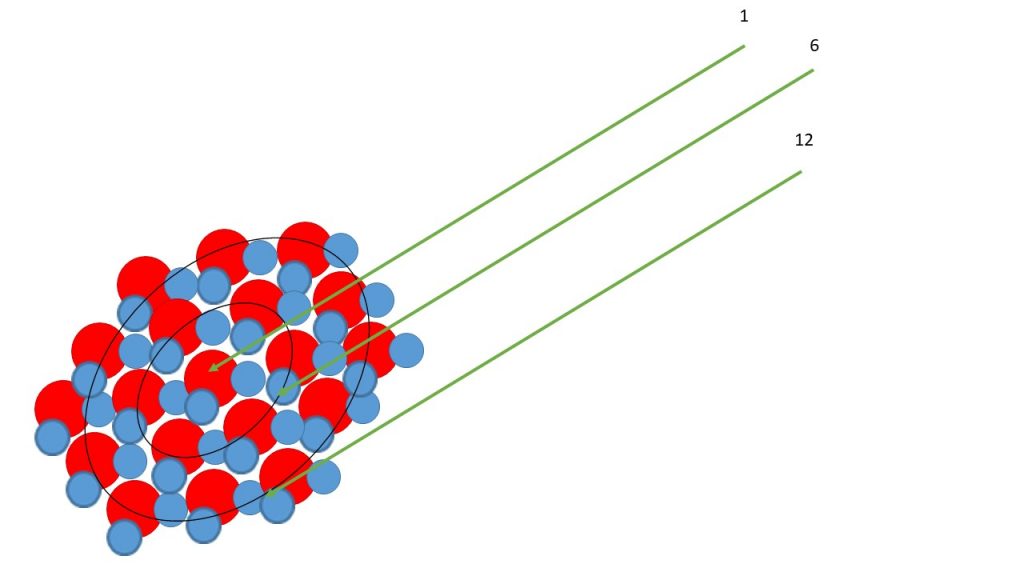
Q: I can’t get the image of the explanation in pp.115, “However, in tiny spherical cloud droplets, there are more molecules in the surface than in the next layer below. Because of this, the intermolecular hydrogen-bond forces acting on the surface molecules are weaker than for a horizontal surface. Thus the vapor pressure at the surface of a very small droplet is higher than given by equation (3.9a) for a horizontal surface, and there is a correspondingly greater tendency for the molecules to evaporate.”
A: Take a look at the schematic view of a water droplet below. The red and blue atoms are H and O, respectively. Count the molecules that make up each ring in the cross section. More energy is needed to create the surface of the small than large droplet. The ratio of the surface (A=4πr2) to volume (V=4/3 π r3) is smaller for a large than small drop.
Acknowledgements
The source of the movie material by Tehani Kuske is the University of Waikato Earth and Ocean Sciences master’s student Tehani Kuske. All rights on this movie are The University of Waikato.
© 2019 Nicole Mölders | All rights reserved
